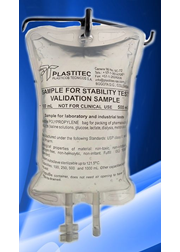
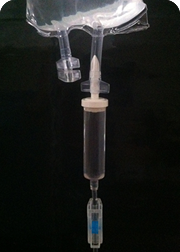
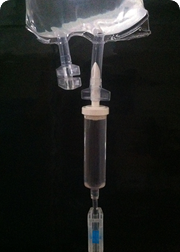
Founded in 1969, Plastitec is mainly focused on the pharmaceutical market by providing accessories, components and packaging materials. Being vertically integrated, we make our own granule compound and have our own processes of extrusion, injection molding, welding, printing and assembly of the product.
The most common finished products are Bags for parenteral solutions (IV), Bags for peritoneal dialysis (CAPD/APD), parenteral nutrition Bags (TPN), Blood Bags, platelet collection Bags and drainage Bags. Furthermore, the company produces a variety of accessories and medical devices.
|
|
|
|
| Professionalism |
Quality |
Innovation |
Technology |